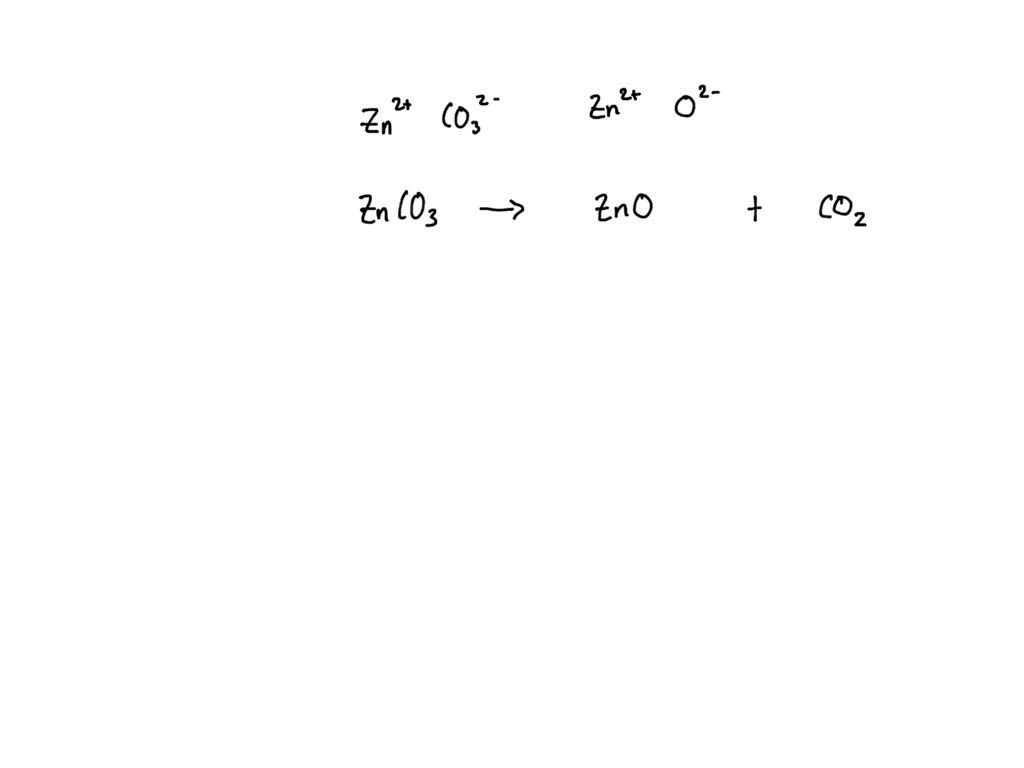Zinc Carbonate Decomposition Equation . For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. The compound named zinc carbonate undergoes decomposition in the presence of heat to form zinc oxide and carbon dioxide. In this video we'll balance the equation znco3 = zno + co2 and provide the correct coefficients for each compound.note:. The general equation for the decomposition of group 2 nitrates is: X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. Zinc carbonate is the inorganic compound with the formula znco 3. 2x (no3)2 (s) 2xo (s) + 4no2 (g). It is a white solid that is insoluble in water. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. It exists in nature as the mineral. Therefore, the chemical equation that shows the thermal. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general form of a. However, other carbonates, such as potassium carbonate, will not thermally.
from www.numerade.com
It exists in nature as the mineral. Zinc carbonate is the inorganic compound with the formula znco 3. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. Therefore, the chemical equation that shows the thermal. However, other carbonates, such as potassium carbonate, will not thermally. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. 2x (no3)2 (s) 2xo (s) + 4no2 (g). It is a white solid that is insoluble in water. In this video we'll balance the equation znco3 = zno + co2 and provide the correct coefficients for each compound.note:.
SOLVED balanced chemical equation for i) Action of an acid on a base
Zinc Carbonate Decomposition Equation Zinc carbonate is the inorganic compound with the formula znco 3. It is a white solid that is insoluble in water. 2x (no3)2 (s) 2xo (s) + 4no2 (g). For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. Therefore, the chemical equation that shows the thermal. The general form of a. Zinc carbonate is the inorganic compound with the formula znco 3. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general equation for the decomposition of group 2 nitrates is: However, other carbonates, such as potassium carbonate, will not thermally. It exists in nature as the mineral. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. In this video we'll balance the equation znco3 = zno + co2 and provide the correct coefficients for each compound.note:. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. The compound named zinc carbonate undergoes decomposition in the presence of heat to form zinc oxide and carbon dioxide.
From gbu-taganskij.ru
What Are Weather Radars And How To Read A Live Weather, 43 OFF Zinc Carbonate Decomposition Equation Therefore, the chemical equation that shows the thermal. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general form of a. The general equation for the decomposition of group 2 nitrates is: 2x (no3)2 (s) 2xo (s) + 4no2 (g). Zinc carbonate is the inorganic compound with the formula znco. Zinc Carbonate Decomposition Equation.
From lexi-bogspotmarsh.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid Zinc Carbonate Decomposition Equation It is a white solid that is insoluble in water. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The compound named zinc carbonate undergoes decomposition in the presence of heat to form zinc oxide and carbon dioxide. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2. Zinc Carbonate Decomposition Equation.
From www.numerade.com
SOLVED 1)Baking soda (NaHCO3) on heating as follows 2 Zinc Carbonate Decomposition Equation A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. Therefore, the chemical equation that shows the thermal. The general equation for the decomposition of group 2 nitrates is: However, other carbonates, such as potassium carbonate, will not thermally. When zinc carbonate is heated, it will decompose to produce zinc oxide, which. Zinc Carbonate Decomposition Equation.
From www.youtube.com
Type of Reaction for ZnCO3 = ZnO + CO2 YouTube Zinc Carbonate Decomposition Equation Zinc carbonate is the inorganic compound with the formula znco 3. Therefore, the chemical equation that shows the thermal. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. It is a white. Zinc Carbonate Decomposition Equation.
From www.numerade.com
12.5g of zinc carbonate is heated, it to make 8.1g of zinc Zinc Carbonate Decomposition Equation Therefore, the chemical equation that shows the thermal. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The general equation for the decomposition of group 2 nitrates is: For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. It is a white. Zinc Carbonate Decomposition Equation.
From www.researchgate.net
(PDF) Thermal of zinc carbonate hydroxide Zinc Carbonate Decomposition Equation A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general form of a. Zinc carbonate is the inorganic compound with the formula znco 3. It exists in nature as the mineral. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon. Zinc Carbonate Decomposition Equation.
From www.slideserve.com
PPT Chapter 8 Review Topics PowerPoint Presentation, free download Zinc Carbonate Decomposition Equation However, other carbonates, such as potassium carbonate, will not thermally. It exists in nature as the mineral. The general equation for the decomposition of group 2 nitrates is: In this video we'll balance the equation znco3 = zno + co2 and provide the correct coefficients for each compound.note:. The compound named zinc carbonate undergoes decomposition in the presence of heat. Zinc Carbonate Decomposition Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Carbonate Decomposition Equation However, other carbonates, such as potassium carbonate, will not thermally. Therefore, the chemical equation that shows the thermal. It is a white solid that is insoluble in water. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. The general equation for the decomposition of group 2 nitrates is: The compound named zinc carbonate undergoes decomposition in the. Zinc Carbonate Decomposition Equation.
From brainly.in
what is the formula of zinc carbonate Brainly.in Zinc Carbonate Decomposition Equation 2x (no3)2 (s) 2xo (s) + 4no2 (g). A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general equation for the decomposition of group 2 nitrates is: Zinc carbonate is the inorganic compound with the formula znco 3. Therefore, the chemical equation that shows the thermal. It is a white. Zinc Carbonate Decomposition Equation.
From www.chegg.com
The of zinc carbonate, ZnCO3(s), into Zinc Carbonate Decomposition Equation In this video we'll balance the equation znco3 = zno + co2 and provide the correct coefficients for each compound.note:. Zinc carbonate is the inorganic compound with the formula znco 3. The general form of a. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. 2x (no3)2 (s) 2xo (s) +. Zinc Carbonate Decomposition Equation.
From testbook.com
Zinc Carbonate Learn its Formula, Structure, Properties & Uses Zinc Carbonate Decomposition Equation The general equation for the decomposition of group 2 nitrates is: For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. In this video we'll balance the equation znco3 = zno + co2. Zinc Carbonate Decomposition Equation.
From www.nagwa.com
Question Video Calculating the Mass of Zinc Carbonate that Will Zinc Carbonate Decomposition Equation The compound named zinc carbonate undergoes decomposition in the presence of heat to form zinc oxide and carbon dioxide. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. It exists in nature as the mineral.. Zinc Carbonate Decomposition Equation.
From studylib.net
Carbonate Zinc Carbonate Decomposition Equation For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. In this video we'll balance the equation znco3 = zno + co2 and provide the correct coefficients for each compound.note:. The compound named zinc carbonate undergoes decomposition in the presence of heat to form zinc oxide and carbon dioxide. The general equation for the. Zinc Carbonate Decomposition Equation.
From www.youtube.com
of Sodium carbonate (balancing equation) YouTube Zinc Carbonate Decomposition Equation For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. Therefore, the chemical equation that shows the thermal. It is a white solid that is insoluble in water. 2x (no3)2 (s) 2xo (s) + 4no2 (g). A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances.. Zinc Carbonate Decomposition Equation.
From www.youtube.com
7.5.2 of Metal Carbonates YouTube Zinc Carbonate Decomposition Equation Therefore, the chemical equation that shows the thermal. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. However, other carbonates, such as potassium carbonate, will not thermally. The general form of a. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. In this video we'll balance the equation znco3 =. Zinc Carbonate Decomposition Equation.
From www.numerade.com
SOLVED Compare the solubility of zinc carbonate in each of the Zinc Carbonate Decomposition Equation However, other carbonates, such as potassium carbonate, will not thermally. The compound named zinc carbonate undergoes decomposition in the presence of heat to form zinc oxide and carbon dioxide. In this video we'll balance the equation znco3 = zno + co2 and provide the correct coefficients for each compound.note:. Zinc carbonate is the inorganic compound with the formula znco 3.. Zinc Carbonate Decomposition Equation.
From sielc.com
Zinc carbonate SIELC Zinc Carbonate Decomposition Equation The compound named zinc carbonate undergoes decomposition in the presence of heat to form zinc oxide and carbon dioxide. The general equation for the decomposition of group 2 nitrates is: For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. A decomposition reaction is a reaction in which a compound breaks down into two. Zinc Carbonate Decomposition Equation.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate Decomposition Equation The general form of a. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. It exists in nature as the mineral. However, other carbonates, such as potassium carbonate, will not thermally. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. For example, copper, lead, and zinc carbonates all. Zinc Carbonate Decomposition Equation.